Enhancing Alzheimer’s Trials with More Responsive Pharmacodynamic Response Biomarkers of Disease Modification
Bridging Pathology and Clinical Outcomes Through Functional Brain Measurement in Early Clinical Development


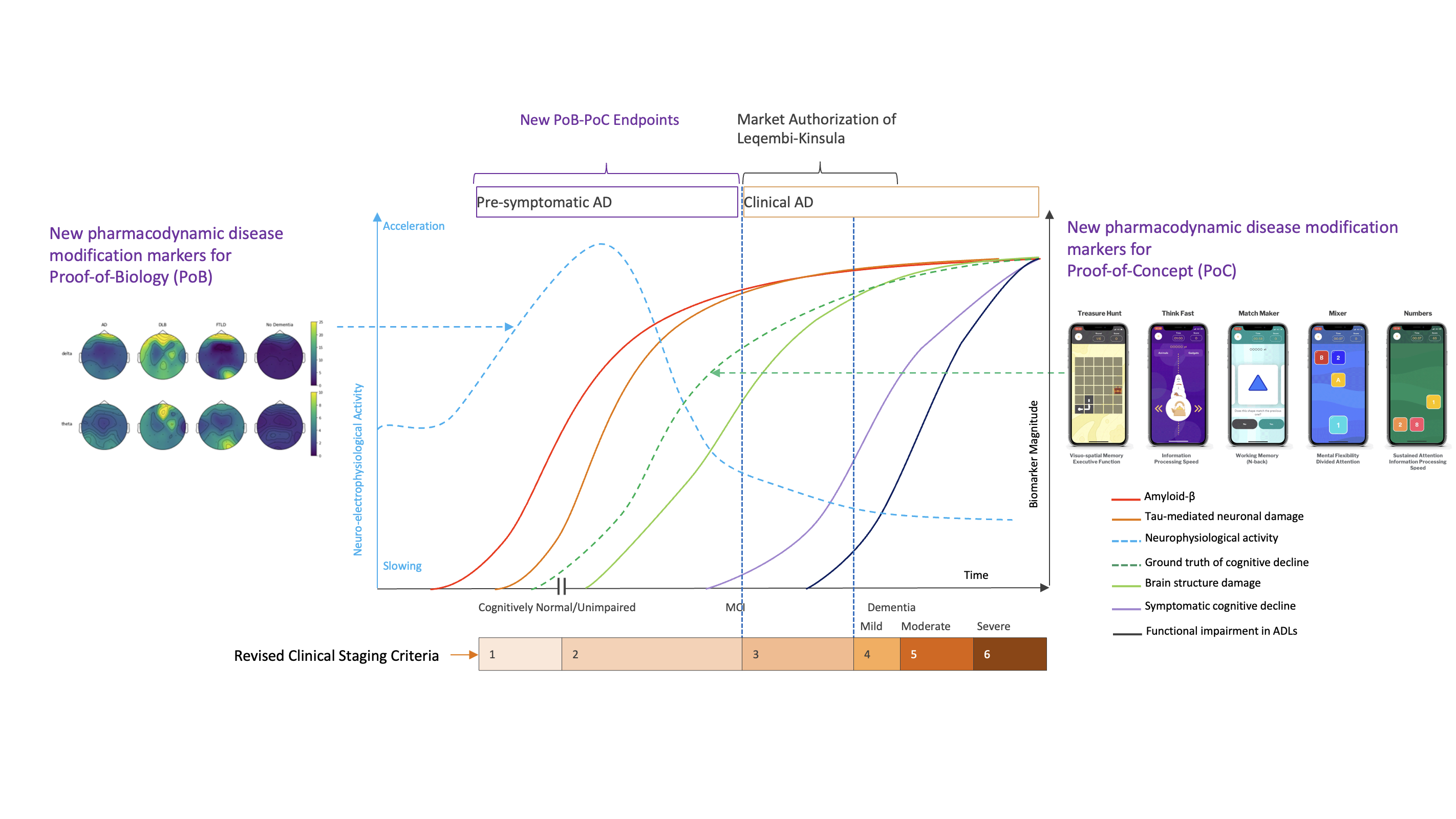
De-risk your clinical development with multimodal insights. By acquiring, aggregating, and analyzing multimodal data from best-matched Digital Health Tech (DHT) solutions, we identify clinically relevant, scientifically validated, commercially viable, and regulatory-accepted endpoints within your specific context of use.
The shared neurobiology of different disorders leads to shared clinical outcomes across different indications.
To measure clinical outcomes within a specific context of use, we need to identify hypothesis-driven and future-proof endpoints.
By selecting the appropriate modalities and matching the right DHTs from our Curated Network of Solutions, we enable the adoption of meaningful endpoints.
Starting from electrophysiology, improving ROI in the process, we help gather insights in CNS. Enabled by our vendor-neutral, data-driven services through our CNS platform.

Match modalities with meaningful endpoints within your context of use.

Select the appropriate DHT partners from our Curated Network of Solutions.

Acquire, aggregate, and analyze multimodal data on our CNS platform.

Discover novel and validate existing CNS endpoints through multimodal insights.

Translate data insights into an actionable clinical development strategy.
A dynamic team of scientists, clinicians, engineers and software developers backed by expert advisors.
Patients impacted
EEGs & PSGs analyzed
Clinical trials enriched
Papers published
Academic and clinical sites
Cliniciansonboarded



Bridging Pathology and Clinical Outcomes Through Functional Brain Measurement in Early Clinical Development






